NERNST EQUATION
NERNST EQUATION :
The relationship between the concentration of ions and electrode potential is given by Nernst equation.
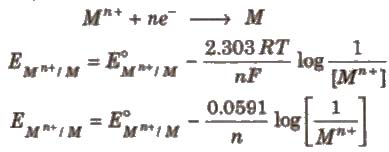
For a electrochemical cell,
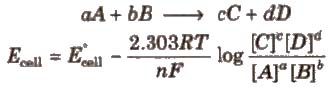
Concentration of pure solids and liquids is taken as unity.
Nernst equation and Kc.
At equilibrium :
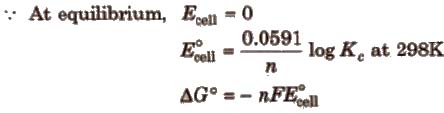
Here, ΔG° is the standard Gibbs free energy change.

Relationship between free energy change and equilibrium constant
ΔG° = – 2.303RT log Kc.

Comments
Post a Comment