ELECTRODE POTENTIAL
E°red = – E°oxidalion
#. STANDARD ELECTRODE POTENTIAL :
The potential difference developed between metal electrode and solution of ions of unit molarity (1M) at 1 atm pressure and 25°C (298 K) is called standard electrode potential.
It is denoted by E°.
@.Reference Electrode
The electrode of known potential is called reference electrode. It may be primary reference electrode like hydrogen electrode or secondary reference electrode like calomel electrode.
Standard hydrogen electrode (SHE) Standard hydrogen electrode (SHE). also known as normal hydrogen electrode (NHE), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. The wire is sealed into a glass tube placed in beaker containing 1 M HCl. The hydrogen gas at 1 atm pressure is bubbled through the solution at 298K. Half-cell is pt H2 (1 atm) H+ (1 M)
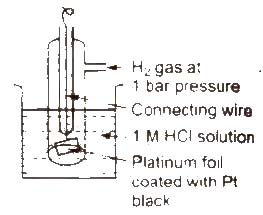
In SHE. at the surface of plantinum, either of the following reaction can take place
2H+(ag) + 2e– → H2G Reduction
H2(g) → 2H+(ag) + 2e– Oxidation
The electrode potential of SHE has been fixed as zero at all temperatures.Its main drawbacks are
- It is difficult to maintain 1 atm pressure of H2 gas.
- It is difficult to maintain H+ ion concentration 1 M.
- The platinum electrode is easily poisoned by traces of impurities.
Hence, calomel electrodes are conveniently used as reference electrodes, It consists of mercury in contact with Hg2 Cl2 (calomel) paste in a solution of KCl.

Comments
Post a Comment